Transition metals and non-ferrous metals such as copper, nickel and cobalt are not only suitable as materials in engineering and technology, but also for a wide range of applications in electrochemistry and catalysis.
October 3, 2022
Transition metals and non-ferrous metals such as copper, nickel and cobalt are not only suitable as materials in engineering and technology, but also for a wide range of applications in electrochemistry and catalysis.
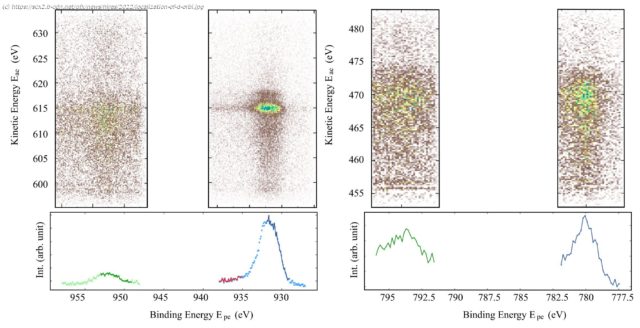
Their chemical and physical properties are related to the occupation of the outer d-orbital shells around the atomic nuclei. The energetic levels of the electrons as well as their localization or delocalization can be studied at the X-ray source BESSY II, which offers powerful synchrotron radiation.
Copper, nickel, cobalt
The team of the Uppsala-Berlin Joint Lab (UBjL) around Prof. Alexander Föhlisch and Prof. Nils Mårtensson has now published new results on copper, nickel and cobalt samples. They confirmed known findings for copper, whose d-electrons are atomically localized, and for nickel, in which localized electrons coexist with delocalized electrons.