Mitochondria—the organelles responsible for energy production in human cells—were once free-living organisms that found their way into early eukaryotic cells over a billion years ago. Since then, they have merged seamlessly with their hosts in a classic example of symbiotic evolution, and now rely on many proteins made in their host cell’s nucleus to function properly.
October 20, 2022
Mitochondria—the organelles responsible for energy production in human cells—were once free-living organisms that found their way into early eukaryotic cells over a billion years ago. Since then, they have merged seamlessly with their hosts in a classic example of symbiotic evolution, and now rely on many proteins made in their host cell’s nucleus to function properly.
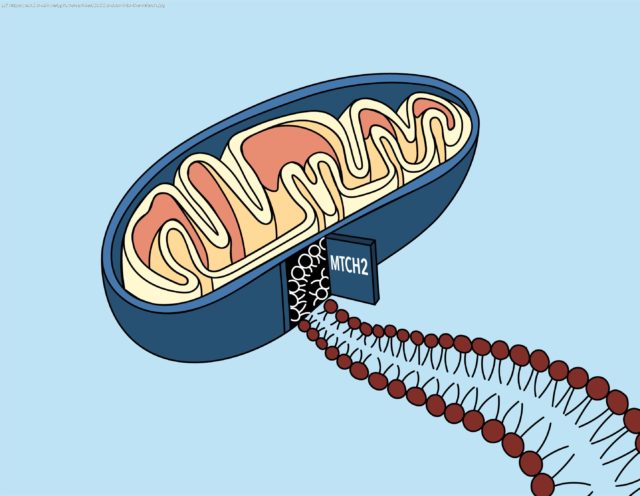
Proteins on the outer membrane of mitochondria are especially important; they allow the mitochondria to communicate with the rest of the cell, and play a role in immune functions and a type of programmed cell death called apoptosis. Over the course of evolution, cells evolved a specific mechanism by which to insert these proteins—which are made in the cell’s cytoplasm—into the mitochondrial membrane. But what that mechanism was, and what cellular players were involved, has long been a mystery.
A new paper from the labs of Whitehead Institute Member Jonathan Weissman and California Institute of Technology professor Rebecca Voorhees provides a solution to that mystery. The work, published October 21 in the journal Science, reveals that a protein called mitochondrial carrier homolog 2, or MTCH2 for short, which has been linked to many cellular processes and even diseases such as cancer and Alzheimer’s, is responsible for acting as a „door“ for a variety of proteins to access the mitochondrial membrane.
„Until now, no one knew what MTCH2 was really doing—they just knew that when you lose it, all these different things happen to the cell,“ said Weissman, who is also a professor of biology at the Massachusetts Institute of Technology and an Investigator of the Howard Hughes Medical Institute. „It was sort of a mystery why this one protein affects so many different processes. This study gives a molecular basis for understanding why MTCH2 was implicated in Alzheimer’s and lipid biosynthesis and mitochondrial fission and fusion: because it was responsible for inserting all these different types of proteins in the membrane.