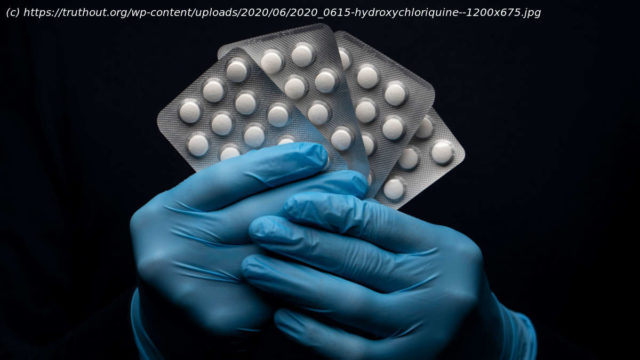
The FDA’s chief scientist said it’s „no longer reasonable to believe“ hydroxychloroquine is effective against COVID-19.
The Food and Drug Administration (FDA) is rescinding its recommendations on use of a drug once touted by President Donald Trump to be a “game-changer” in the fight against coronavirus.
Hydroxychloroquine was previously given “emergency use” authorization for patients hoping it could help in the treatment of the disease. That status was removed in an announcement from the agency on Monday.
FDA chief scientist Denise Hinton, writing to Gary Disbrow of the Biomedical Advanced Research and Development Authority, announced that “it is no longer reasonable to believe that oral formulations of HCQ [hydroxychloroquine] and CQ [chloroquine] may be effective in treating COVID-19, nor is it reasonable to believe that the known and potential benefits of these products outweigh their known and potential risks.”
“Accordingly, FDA revokes the EUA for emergency use of HCQ and CQ to treat COVID-19,” Hinton added.
Trump had been touting hydroxychloroquine for months, recommending it without having scientific backing to support his claims on its efficacy.
“I’m seeing people dying without it…. When that’s happening, they should do it.
Start
United States
USA — Science FDA Strips “Emergency Use” Authorization for Trump’s “Game-Changer” COVID Drug