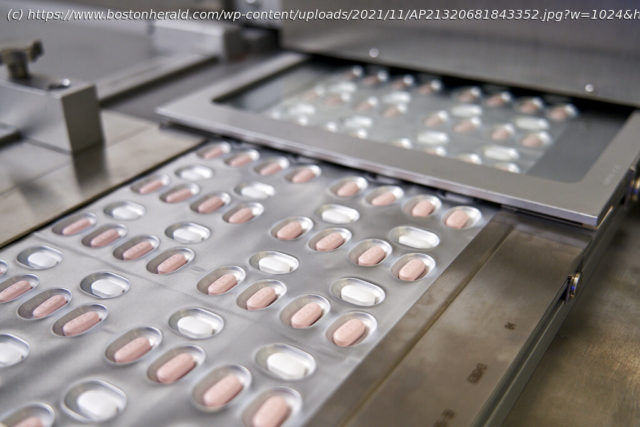
Pfizer asked U.S. regulators Tuesday to authorize its experimental pill for COVID-19, setting the stage for a likely launch this winter of a promising treatment that can be taken at home.
WASHINGTON — Pfizer asked U.S. regulators Tuesday to authorize its experimental pill for COVID-19, setting the stage for a likely launch this winter of a promising treatment that can be taken at home. The company’s filing comes as new infections are rising once again in the United States, driven mainly by hot spots in states where colder weather is driving more Americans indoors. Pfizer’s pill has been shown to significantly cut the rate of hospitalizations and deaths among people with coronavirus infections. The Food and Drug Administration is already reviewing a competing pill from Merck and several smaller drugmakers are also expected to seek authorization for their own antiviral pills in the coming months. “We are moving as quickly as possible in our effort to get this potential treatment into the hands of patients, and we look forward to working with the U.