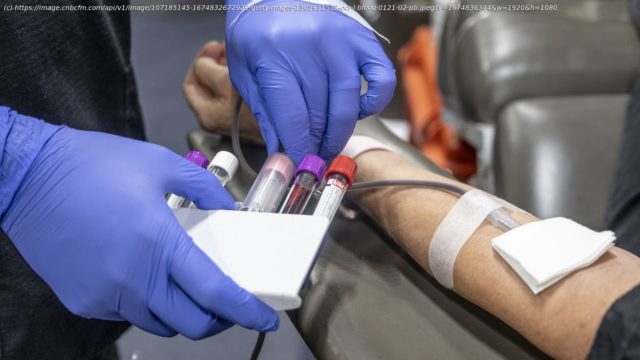
The FDA said the policy would shift to an individual assessment that evaluates an individual’s risk regardless of gender sexual orientation.
The Food and Drug Administration on Friday proposed new guidelines that would no longer require gay and bisexual men in monogamous relationships to abstain from sex before donating blood.
The FDA had imposed a lifetime ban on men who have sex with men donating blood during the AIDS crisis in the 1980s. The agency had eased the ban in 2015, allowing gay and bisexual men to donate blood if they had not had sex in the previous year.
In response to a blood donor shortage during the Covid pandemic, the FDA further eased restrictions in April 2020 to allow gay and bisexual men who had not had sex in the past three months to donate.
Under the guidelines proposed on Friday, gay and bisexual men who are in monogamous relationships would be allowed to donate blood. But individuals, regardless of gender or sexual orientation, who have recently had anal sex with a new or multiple partners would have to wait three months before donating.
« Maintaining a safe and adequate supply of blood and blood products in the U.
Home
United States
USA — Science FDA proposal would allow gay men in monogamous relationships to donate blood