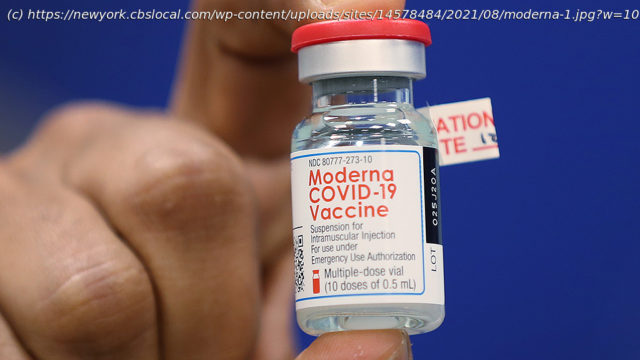
The Food and Drug Administration Advisory Committee considered extensive data on the safety and effectiveness of the Moderna mRNA vaccine for experimental use authorization.
NEW YORK (CBSNewYork) — The Food and Drug Administration on Thursday approved emergency use of a third dose of the Moderna COVID-19 vaccine, for certain groups of Americans. The agency’s advisory committee considered extensive data on the safety and effectiveness of the Moderna mRNA vaccine for experimental use authorization. That authorization has already been granted for limited recipient groups to the Pfizer vaccine last month.
Домой
United States
USA — Political FDA Panel Approves Experimental Use Authorization Application For Moderna COVID-19 Booster Shots...