The FDA approved two new therapies for sickle-cell disease, including the world’s first-ever approved CRISPR therapy.
In a historic first, the Food and Drug Administration (FDA) has approved America’s first gene therapies for sickle-cell disease (SCD), one of which uses the gene-editing tool CRISPR.
«Sickle cell disease is a rare, debilitating and life-threatening blood disorder with significant unmet need [for better, long-lasting treatments], and we are excited to advance the field especially for individuals whose lives have been severely disrupted by the disease by approving two cell-based gene therapies today,» Dr. Nicole Verdun, director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research, said in a statement released Friday (Dec. 8).
«Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited,» she said.
The U.K. became the first country to approve the CRISPR-based therapy, called Casgevy, in mid-November. Experts anticipated that the FDA would soon echo the decision made by U.K. regulators, as advisors to the FDA had deemed the treatment safe for clinical use back in October.
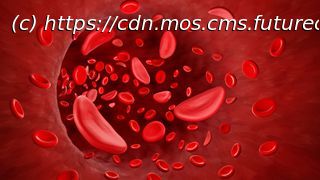
SCD is caused by genetic mutations that change the shape of the protein hemoglobin, which carries oxygen in red blood cells. Red blood cells then become sickle-shaped, rather than round, which causes them to die off quickly and also stick together, blocking blood vessels.
Домой
United States
USA — IT 1st gene therapies for sickle cell cleared by FDA, including CRISPR treatment