In nature, there are animals that make fibers that are strong and elastic—for example, the thread that spiders produce to make webs. These fibers have a polypeptide structure and serve as inspiration for research into the development of functional materials.
In nature, there are animals that make fibers that are strong and elastic—for example, the thread that spiders produce to make webs. These fibers have a polypeptide structure and serve as inspiration for research into the development of functional materials.
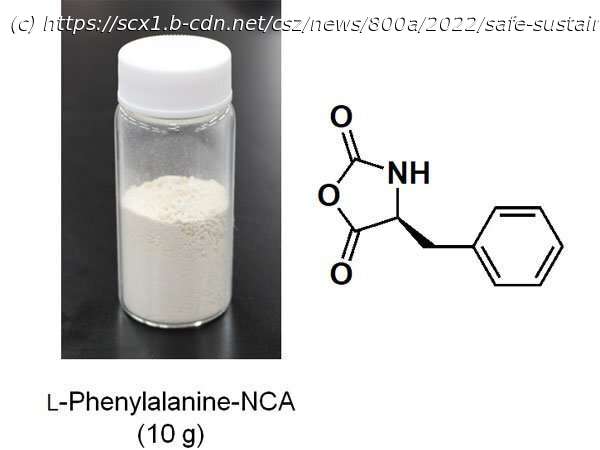
Alpha (α)-amino acid N-carboxyanhydrides (NCAs) are precursors for artificial polypeptides. However, this compound decomposes easily, making it difficult to obtain commercially. Therefore, it is necessary to synthesize the right quantity of α-amino acid NCAs at the location and time that they are required.
NCAs are usually synthesized from plant-derived amino acids and phosgene. However, phosgene is extremely toxic and dangerous to use, leading to growing demand for new chemical compounds and reactions that can be substituted for it. Using the photo-on-demand phosgenation method that they previously developed, Associate Professor TSUDA Akihiko’s research group at Kobe University’s Graduate School of Science has succeeded in synthesizing NCA in a safe, inexpensive and simple manner from chloroform (a common organic solvent) and amino acid.
The academic paper was published online in ACS Omega on October 19, 2022.
Phosgene (COCl2) is used as precursor for polymers and as a pharmaceutical intermediate. The global phosgene market continues to grow by several percent each year, with around 8 to 9 million tons produced annually. However, phosgene is extremely toxic. For safety reasons, research and development is being conducted to find alternatives.
In a world-first discovery, Associate Professor Tsuda’s research group irradiated chloroform with ultraviolet light, which caused it to react with oxygen and generate high yields of phosgene (patent no. 5900920). In order to do this in an even safer and easier manner, the research group found a way that the phosgene-generating reactions could be instantly performed.