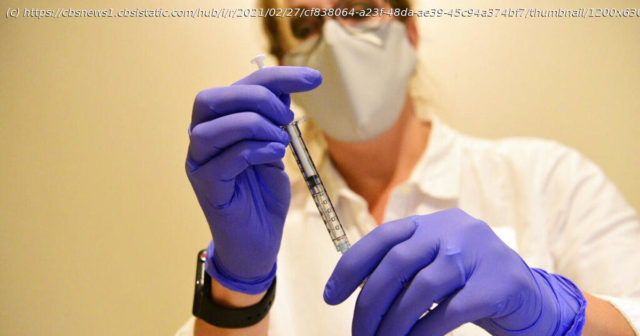
The vaccine is the third to be approved for use in the United States, and the first that requires only one shot.
The Food and Drug Administration on Saturday authorized Johnson & Johnson’s COVID-19 vaccine for emergency use. The vaccine is the third to be approved for use in the United States, and the first that requires only one shot. The FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC) voted unanimously to approve the vaccine by Janssen, a division of Johnson & Johnson, on Friday. The committee provides expert advice to the FDA but does not have final say on approval. « The authorization of this vaccine expands the availability of vaccines, the best medical prevention method for COVID-19, to help us in the fight against this pandemic, which has claimed over half a million lives in the United States, » said Acting FDA Commissioner Dr. Janet Woodcock on Saturday. « The FDA, through our open and transparent scientific review process, has now authorized three COVID-19 vaccines with the urgency called for during this pandemic, using the agency’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization. » The Centers for Disease Control and Prevention updated state and local partners on distribution plans for the vaccine on Friday, ahead of the FDA’s authorization and VRBPAC’s approval.
Home
United States
USA — Political Johnson & Johnson's one-shot COVID vaccine authorized for emergency use