Array
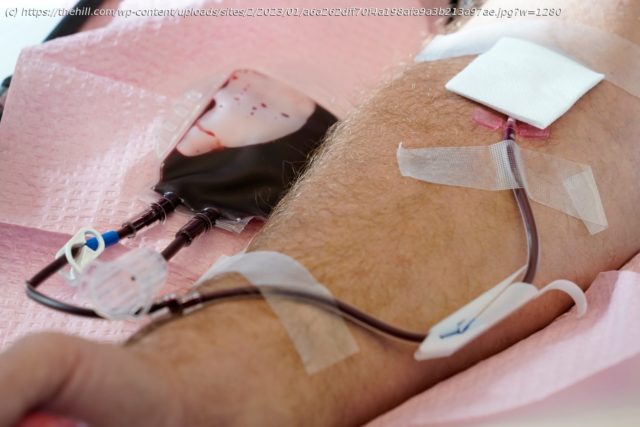
The Food and Drug Administration (FDA) has issued draft guidance to change its policies on donating blood, moving away from time-based deferrals for men who have sex with men and instead proposing “individual risk-based questions” to reduce the potential spread of HIV through transfusions.
Under current FDA guidelines for donating blood, men who have sex with men are permitted to donate blood after a three-month deferral period in which they abstain from sexual encounters with men. This change was made in 2020, after the previous guidance had mandated a 12-month deferral period.
The new question-based approach would instead ask potential donors about new or multiple sex partners they have had in the past three months.
Home
United States
USA — Financial FDA drafts new ‘gender-inclusive’ guidance on blood donation eligibility, HIV risk reduction