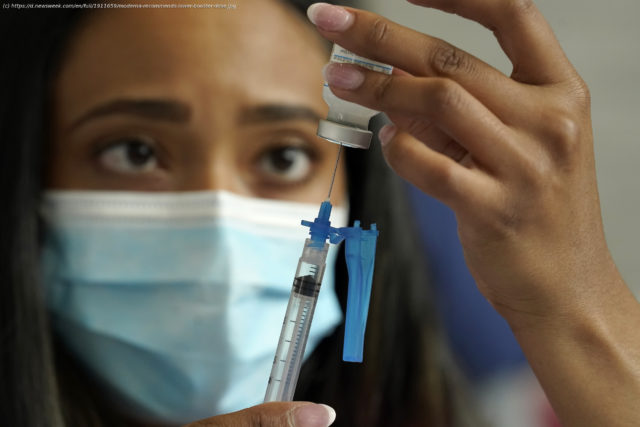
Moderna said that study subjects who received half the dose of a regular shot saw a jump in virus-fighting antibodies.
As the Food and Drug Administration’s independent advisers are set to begin considering booster shots from COVID-19 vaccine manufacturers other than Pfizer, Moderna is recommending that healthy Americans receive half the dose of its regular vaccine shot, the Associated Press reported. The first two Moderna doses inject recipients with 100 micrograms of the vaccine, but the company says that just 50 grams in a booster should be sufficient. Moderna conducted a study with 344 people, all of whom received a shot with 50 micrograms six months after their second vaccine dose. The company said that recipients saw a jump in virus-fighting antibodies, as well as a 42-fold increase in antibodies that can battle the Delta variant of the virus, the AP reported. The study subjects saw similar side effects as those commonly experienced after the second shot of the vaccine, including fevers and aches. The FDA ‘s advisers on Thursday will begin considering the authorization of a third Moderna booster, as well as a booster for the one-dose Johnson & Johnson vaccine, per the AP. For more reporting from the Associated Press, see below. The final go-ahead for the two vaccine boosters is not expected for at least another week. After the FDA advisers give their recommendation, the agency itself will make a decision on whether to authorize boosters. Then next week, a panel convened by the Centers for Disease Control and Prevention will offer more specifics on who should get them. Its decision is subject to approval by the CDC director. The process is meant to bolster public confidence in the vaccines. But it has already led to conflicts and disagreements among experts and agencies. For example, last month the CDC advisory panel backed Pfizer boosters at the six-month point for older Americans, nursing home residents and people with underlying health problems.
Домой
United States
USA — Science Moderna Suggests FDA Approve Lower Dose COVID Booster Shot for Healthy Americans